Two principles that we at Scientific Update teach in the foundational “Chemical Development” course are that new experimental methods can open new opportunities for old reactions, and that as scientists we should be looking to understand the mechanisms of empirical observations. Both of these principles are exemplified in by a recent pre-print publication by the Baran team on new reaction conditions for the venerable Kolbe Coupling (Overcoming the Limitations of Kolbe Coupling via Waveform-Controlled Electrosynthesis, DOI: 10.26434/chemrxiv-2022-3cj82-v2)
The electrochemical coupling of two carboxylic acids to form a new C-C bond is almost 200 years old having been initially reported by Faraday in 1834 before being investigated and reported in more detail by Kolbe in 1849. I remember the reaction being presented briefly in undergraduate lectures and as something I needed to know in case it came up in final exams, but in over 25 years of synthetic chemistry research I don’t think I ever heard anyone suggest it as an approach to make our of our target molecules, because the other fact that I remember from lectures was that the reaction conditions were harsh and that functional group tolerance was poor. Also, synthetic electrochemistry was rarely practiced by non-experts until only a few years ago, and there were so many ‘better’ ways to make C-C bonds using metal-mediated cross-coupling reactions! However, in recent years there has been a renaissance in synthetic electrochemistry that make this a tool for generalists as well as a growing awareness of the long-term unsustainability of relying solely on expensive and frequently toxic transition metals to make C-C bonds. Many carboxylic acid building blocks can be readily obtained from biomass sources rather than petrochemicals, so reinvigorating the Kolbe reaction would provide a new source of feedstocks that could be transformed to higher value materials using electrons which are increasingly available from renewable energy technologies.
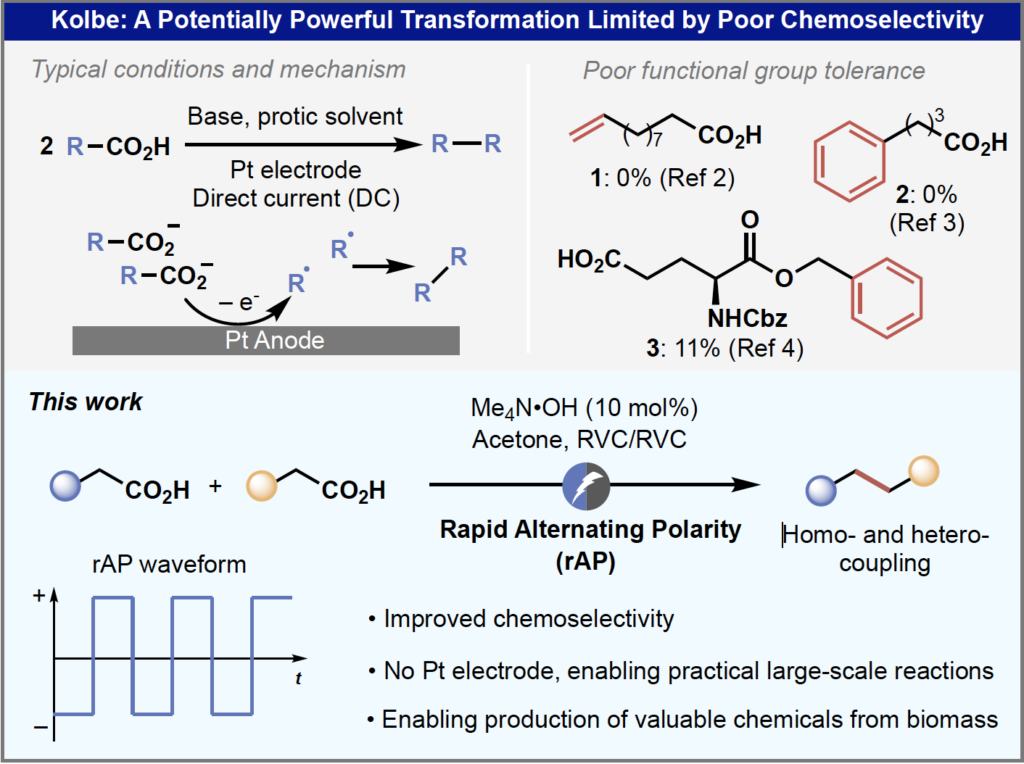
In the past couple of years, the Baran team have reported two fascinating papers where they applied rapidly alternating polarity (rAP) to enhance the selectivity of reductive electrochemical reactions. Briefly, rAP involves a rapid square-waveform reversal of the electrochemical cell anode and cathode on millisecond timescales. The initial paper (J. Am. Chem. Soc. 2021, 143, p. 16580) describes a series of selective electrochemical carbonyl reductions on highly functionalized substrates that typically afforded complex reaction mixtures using conventional synthetic electrochemistry conditions. In this case the rAP waveform was proposed to work by favoring the initial fast reduction over slower secondary reductions. The second paper (J. Am. Chem. Soc. 2022, 144, p. 5762) extended the rAP approach to the Birch reduction so that liquid ammonia (my least favorite solvent in graduate school) is no longer needed. In this case mechanistic studies were consistent with the benefits of the rAP waveform coming from suppression of competing proton-reduction pathways.
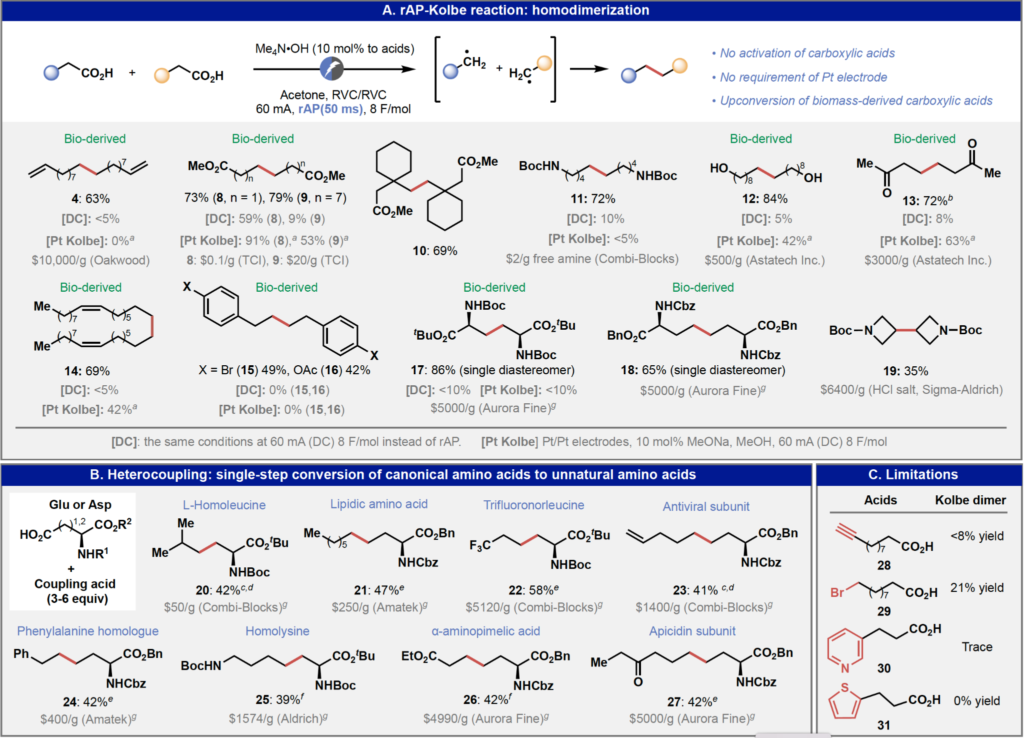
This latest paper on the rAP enabled Kolbe coupling starts by describing the initial reaction condition screening studies using 10-undecenoic acid, a substrate that is incompatible with the classical Kolbe reaction conditions: direct current, Pt electrodes, methanol solvent with 10 mol % NaOMe. Interestingly Kolbe coupling of this substrate had been studied in 1939 using standard alternating current with Pt electrodes and shown to be unproductive; a result which was verified in this current paper. However, the value of being persistent and willing to apply new reaction conditions and techniques to old reactions was amply (pun intended) demonstrated when the Baran team switched to a rAP waveform with 50 ms pulse width (10 Hz) using carbon-based electrode to afford the desired product in 63% isolated yield! The authors note that the fact that carbon electrodes can be used is particularly surprising given that Pt electrodes have been considered to be essential for over a century for successful Kolbe electrolysis. Acetone is favored as a solvent, presumably for its ability to accept electrons and protons thereby balancing oxidative decarboxylation, and this is supported by the detection of isopropanol in the reaction mixture by 1H NMR spectroscopy.
The wide functional group tolerance of the rAP-enabled Kolbe reaction is demonstrated by the examples in the figure below. Although at first glance many of the yields might be viewed as ‘moderate to good’ rather than ‘excellent’ the worth of the Kolbe approach is the synthetic complexity reduction enabled by being able to take inexpensive materials (many bio-derived) and upgrade them to much more valuable materials in a single step. I particularly appreciated the supporting information including a review section on previous methods to deliver many of these materials which demonstrates the difference between this simple single-step Kolbe coupling approach and the complex multi-step procedures otherwise needed.
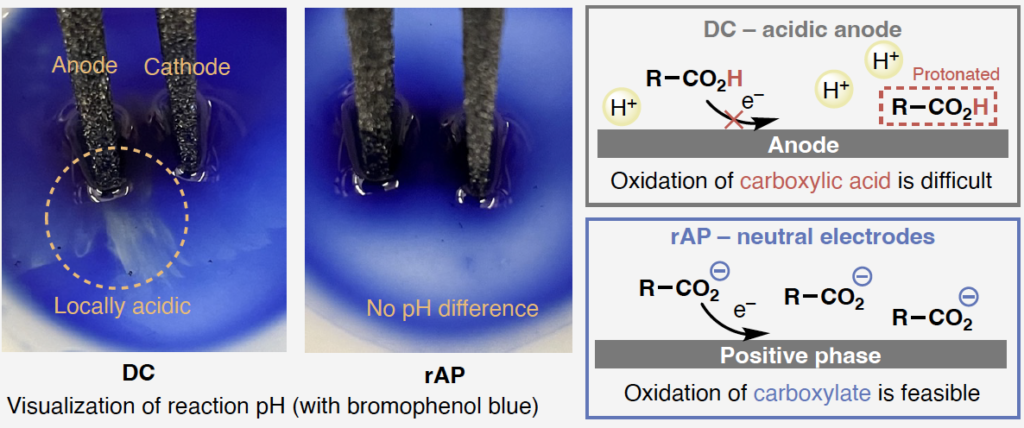
Unexpected results in chemistry, and especially during scale-up, are often explained by consideration of localized and/or temporal effects. In this paper the authors present strong evidence that the unexpectedly good results produced by the rAP waveform result from changing the local acidity at the anode. Under standard DC current conditions a localized area of enhanced acidity forms at the anode at the microscopic level regardless of stirring rate. This leads to protonation of the carboxylate substrate as it approaches anode which in turn makes oxidation difficult. The localized build-up of acidity around the anode is clearly shown by adding bromophenol blue to the reaction mixture (below left) whereas the rAP oscillations prevent the buildup of a persistent acid layer (below center) and the substrate can reach the anode as the carboxylate form where it is preferentially oxidized. This result is further confirmed by adding an acid labile substrate to the reaction mixture and observing significant deprotection under standard DC conditions but not under rAP conditions.
As a process chemist I’m always most interested in whether academic groups have exemplified their new synthesis method on at least multi-gram scale and the Baran team, as usual, don’t disappoint. Two of the biomass derived materials were scaled up to 200-300 mmol scale, but I was particularly impressed by the large-scale (25 g) heterocoupling given that the product is the type of unnatural amino acid frequently encountered in the pharmaceutical industry but often expensive and not immediately available on the scale required. The detailed procedure for the large scale reaction is provided in the supporting material and was performed in a widely-used commercial automated jacketed reactor system demonstrating that this technology should be readily transferable to process development and scale-up laboratories.
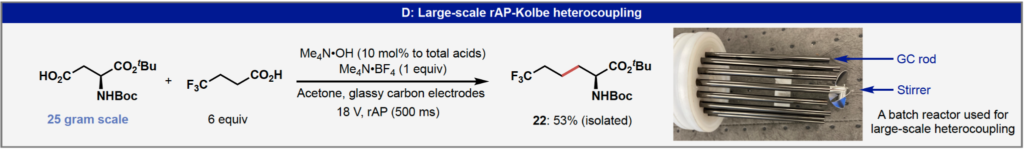
Interestingly this heterocoupling scale-up reaction is performed with a rAP pulse width of 500 ms which is 10-fold longer than the standard conditions. Unfortunately I can find no direct comment in either the main text or supporting information from the authors as to why they made this change for scale-up, although a note in the supporting information does mention that a longer pulse does lead to higher conversion for the same current but this can be at the cost of increased risk of side reactions and electrode passivation. Regardless, this rAP-enabled of modification of the Kolbe coupling is a significant improvement of this classic C-C bond-forming method and will likely soon find utility in many laboratories focused on rapidly converting simple starting materials into complex and valuable materials under relatively sustainable reaction conditions.








