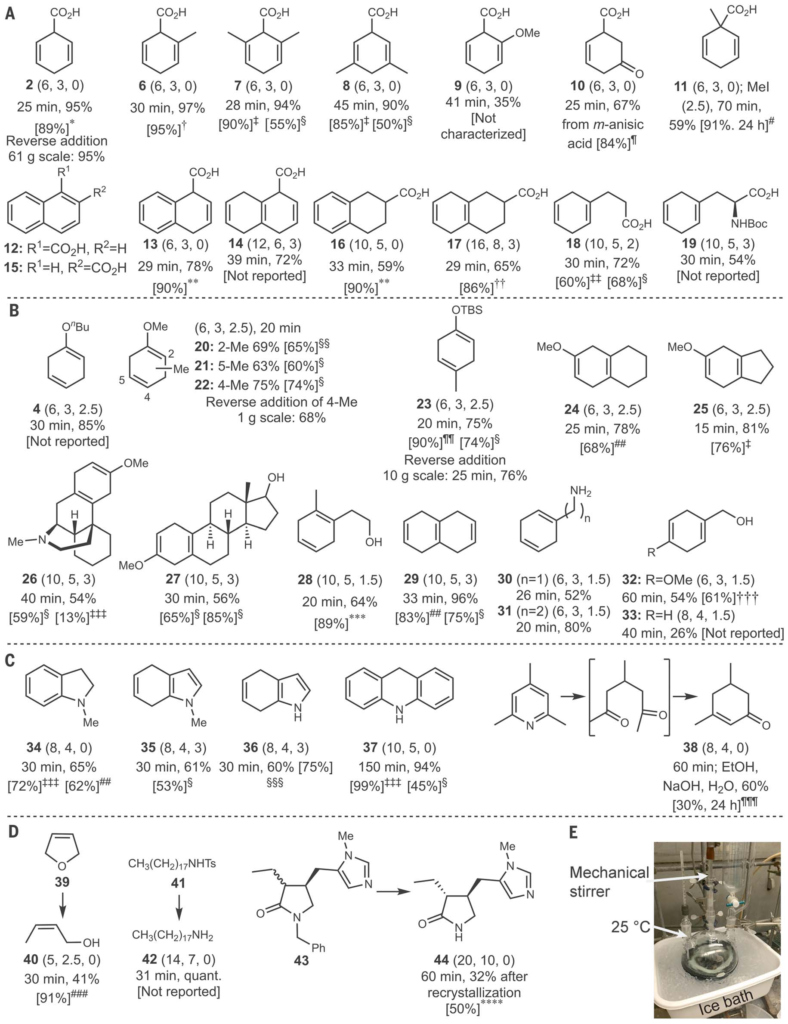
The Birch reduction is a great reaction on small scale and can be carried out on large scale but requires specialist equipment to handle liquid ammonia (bpt -33°C) and it takes a significant amount of time to evaporate the ammonia at the end of the reaction.
There have been various reports of alternative methods using for example ethylamine as an alternative to liquid ammonia, but the methods are often limited to particular substrate classes.
This new method is quick to carry out and requires the addition of lithium metal to the substrate in ethylenediamine and THF. Work up involves quenching with water, evaporation of THF and then partition between concentrated aqueous HCl and ethyl acetate.
The reaction has been scaled up to 10 g scale, not massive by process chemistry standards, but very respectable for an academic lab. The only disappointment is that you have to use lithium as sodium does not work.
- Burrows, S. Kamo and K. Kiode, Science, 2021, 374, 741-746.