Perhaps the best-known ring-expansion reaction is the Baeyer-Villiger oxidation, first reported in 1899- not surprisingly by Adolf von Bayer and Victor Villiger. As we’re all aware, reaction of a carbonyl with a peracid generates a Criegee-type intermediate that undergoes rate-limiting concerted rearrangement to form an ester or lactone. The migration order is substituent dependant, with the most electron-rich alkyl group (more substituted carbon) migrating first. The general migration order is tertiary alkyl > cyclohexyl > secondary alkyl > benzyl > phenyl > primary alkyl > methyl >> H. Asymmetric variations, either through kinetic resolution of racemic cycloketones or desymmetrization of mesomeric cycloketones, are well known.1
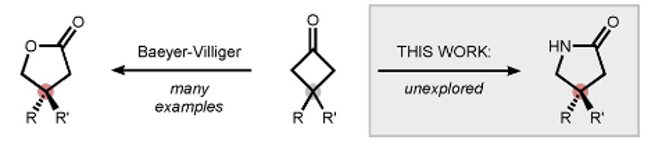
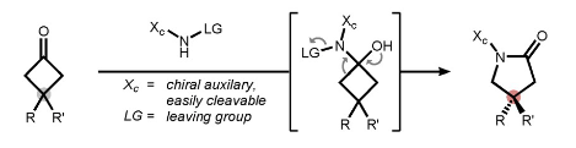
A recent paper by Wahl et al in Angewandte Chemie, though not strictly a Baeyer-Villiger reaction, describes a related asymmetric nitrogen-based ring expansion, that leads to chiral γ-lactams (Figure 1).2 This structural motif frequently finds its way into chemical matter emerging from drug discovery programmes and indeed marketed drugs. Examples include carmegliptin,3a rolipram,3b and brivaracetam.3c,d The synthetic approach described in the paper is actually based on the classical Beckmann reaction- utilizing a bi-functional amine bearing a suitable leaving group and a chiral auxiliary (Figure 2). Due to inherent ring strain the intermediate hemiacetal undergos ring expansion to the lactam (Figure 1). Cyclobutanones have the right amount of inherent ring stain to promote the desymmetrization reaction. The chemistry of these intriguing intermediates has been reviewed.4
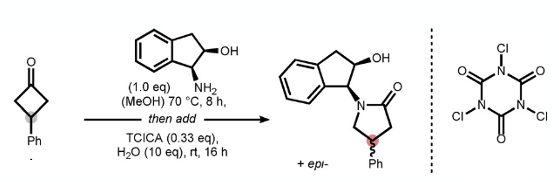
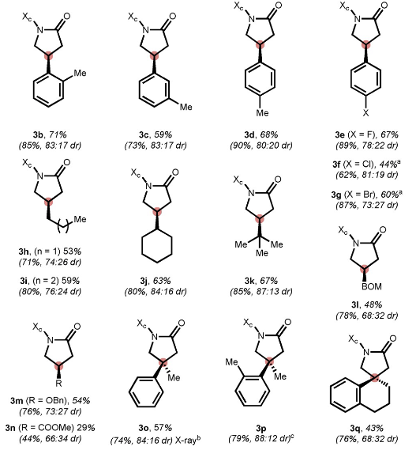
Suitable chiral amines (auxiliary Xc) and appropriate halogen sources (leaving group LG) were screened and an indanol / trichloroisocyanuric acid combination worked well in the trial experiments using 3-phenylcyclobutanone (Figure 3). The overall yield was high (96%) with a diastereomeric ratio of 80:20. Plenty of examples are provided in the paper (Figure 3)
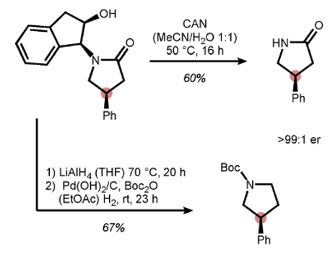
A couple of approaches for removal of the indanyl are described. Surprisingly direct hydrogenation of the lactam product was unsuccessful. Lactam reduction using LiAlH4 followed by hydrogenolysis and Boc- protection gave the protected pyrrolidine product. Ceric ammonium nitrate (CAN) gave the required lactam (exemplified with 4-phenyl model substrate) in somewhat disappointing 60% yield (Figure 4). The hydroxy group in the axillary was vital for the success of the cleavage reaction. Birch reduction conditions would also most likely work but are really a sledgehammer to crack a walnut.
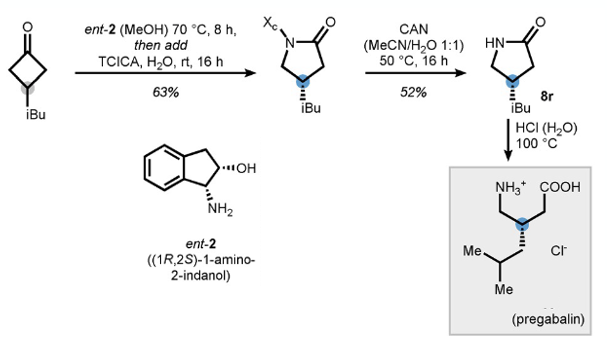
Pulling everything together the authors describe a short synthesis of pregabalin from 4-i-butylcyclobutane and the (1R,2S) isomer of 1-amino-2-indanol (Figure 5). Rearrangement gave the isolated lactam in 63% yield. CAN cleavage of the auxiliary followed by hydrolysis with aqueous HCl (100°C) gave pregabalin.5
A significant chunk of the paper is dedicated to understanding the observed stereochemical induction using computational and spectroscopic methods.
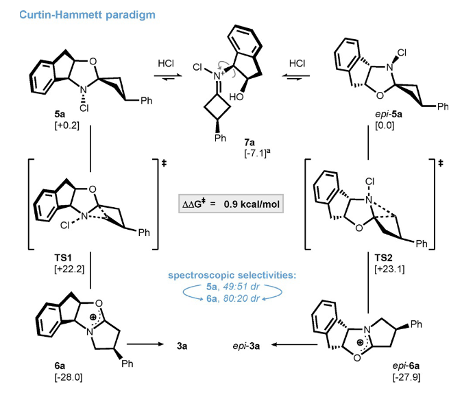
Using NMR spectroscopy the formation of 5a and its epimer (5a-epi) and the downstream carbenium ions 6a/6a-epi was monitored (Figure 6). An initial ratio of 49:51dr was observed for the equilibrating chloroamines, increasing to 80:20 dr for the oxocarbenium species. These results were rationalised by invoking the Curtin-Hammett principle. Interconversion of the chloroamines 5a/epi-5a via the iminium species 7a (formed from HCl protonation of the oxygen and ring-opening) showed a calculated energy difference of only 0.2 Kcal/mol-1 consistent with rapid equilibration and an expected 49:51 rato.6 However the stereo-differentiating transition state energy differences were calculated at 0.9 Kcal/mol-1, translating to the observed downstream 80:20 selectivity. The ring-stain inherent in the cyclobutanone ring is key to driving the re-arrangement.
An interesting paper and a novel way of preparing γ-lactams, including those bearing all-carbon quaternary stereocenters.
See you next time.
References:
- Asymmetric Baeyer-Villiger oxidation: classical and parallel kinetic resolution of 3-substituted cyclohexanones and desymmetrization of: meso-disubstituted cycloketones, X. Liu et al, Sci. 2019, 10, 7003-7008.
- Desymmetrization of prochiral cyclobutanones via nitrogen insertion: a concise route to chiral γ-lactams, J. Wahl et al, Chem.Int.Ed. ,2021, 60, 9719–9723.
- a) An efficient process for the manufacture of carmegliptin, A. Fettes et al, Process Res. Dev.2011, 15, 503–514; b) Efficient synthesis of (−)-(R)- and (+)-(S)-rolipram, R. Kaur et al, Tett. Lett. 2017, 58, 4333-4335; c) Synthetic approaches toward the synthesis of brivaracetam: an antiepileptic drug, G. Eppa et al, ACS Omega 2022, 7, 2486-2503; d) Recent advances in chemistry of gamma-lactams: part i. synthesis starting from acyclic or cyclic precursors, S. Rinaldi et al, Curr. Org. Chem. 2014, 18, 1373-1481.
- Enantioselective desymmetrization of cyclobutanones: a speedway to molecular complexity, M. Wahl et al, Angew. Chem. Int. Ed. 2020, 59, 6964 – 6974
- Process for the preparation of pregabalin, EP2418194
- Energies were obtained by using the following method:PW6B95-D3//TPSS-D3/def2-TZVP+COSMO-RS.








