A new paradigm in drug development began in the spring of last year with the Food and Drug Administration’s (FDA’s) approval of Teva pharmaceuticals’ VMAT2 inhibitor deutetrabenazine as a new chemical entity (NCE) under its 501(b)(2) legislation. This molecule is unique in that it is the first deuterium-substituted drug to reach the market and heralds a new era for clinical development of similarly modified medicines.1
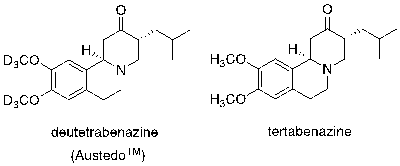
The science behind this approach is relatively simple. The deuterium atom is heavier than protium (with one additional neutron in its nucleus) and as a result its bond to carbon is stronger. The consequence is a difference in the rate of any bond breaking reaction step (a primary kinetic isotope effect), where the C-D bond breaks more slowly than the corresponding C-H bond.2 These effects are generally used in organic synthesis as probes to prove or disprove reaction mechanisms. Since metabolic processing of drug molecules in vivo is really just a chemical transformation akin to any other reaction one might observe, the same kinetic effects should be apparent. The consequence for a drug molecule is that slowing the rate of metabolism, i.e. promoting a longer half-life, can extend the duration of therapeutic drug concentrations in plasma for a given dose- an obvious benefit for the patient. In addition, introduction of deuterium in place of protium at a stereogenic centre within a drug molecule can, in theory, slow the rate of in vivo epimerisation or racemisation of a vulnerable chiral centre and stabilize one isomeric form of the molecule. The approval of deutetrabenazine shows a clinical validation of the mechanism and safety of the (metabolism slowing) approach, at least in the context of this particular API.3 Replacement of the two methoxy groups in the original molecule tetrabenazine with tridueteromethoxy improves tolerability and facilitates an improved dosing regimen. Metabolic demethylation in vivo is slowed by incorporation of the deuterium isotope in place of protium. AustedoTM is marketed as a racemate and is used to treat chorea, a movement disorder comorbid with Huntington’s disease, and tardive dyskinesia. Tetrabenazine was discovered in the 1950’s and approved by the FDA for chorea in 2008. The deuterated version has a twice-daily dosing regimen instead of three times per day and requires a lower overall daily dose.
Most major pharma companies have at least one deuterated molecule in their development portfolio, many in late stage clinical trials. Examples include ivacaftor (CTP-656), dextromethorphan (AVP-786) and linoleic acid (RT001). With a clearer roadmap to navigate the regulatory highway and potentially less resistance from the FDA there should be an increase in the number of deuterated molecules entering development over the coming years.
Manufacture of deuterated molecules and intermediates is obviously an important factor in the development process. Introduction of deuterium into a molecule is not always straightforward and can be complicated by low levels of incorporation or protium/deuterium exchange during synthesis. Availability and supply of deuterated starting materials, particularly for manufacture of API’s at reasonable cost, are also important considerations. A number of new technologies have been developed over the last few years to improve synthesis efficiency and this continues to be an active and exciting area of research.
To find out more about the role of deuterium in drug discovery/development, how to synthesize deuterated compounds and intermediates or just to keep abreast of this rapidly evolving area sign up for the FREE Scientific Update webinar: Click HERE
1) Chem. Eng. News 2016, 94(27), 32-36; Biochemistry 2018, 57, 472-473
2) J. Med. Chem. 2014, 57, 3595-3611
3) J. Clinical Movement Disorders 2017, 4, 11








